A Phos-tag-based fluorescence resonance energy transfer system for the analysis of the kinase reaction of a substrate peptide
Abstract
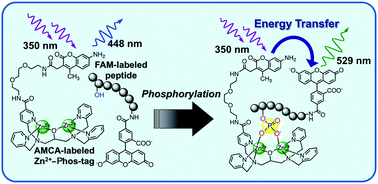
We recently reported a fluorescence resonance energy transfer (FRET) system for the analysis of the dephosphorylation of a 5-carboxyfluorescein (FAM)-labeled phosphopeptide by using a Phos-tag (1,3-bis[bis(pyridin-2-ylmethyl)amino]propan-2-olato dizinc(II) complex) derivative attached to a (7-amino-4-methylcoumarin-3-yl)acetic acid (AMCA) moiety. This FRET system is based on the principle that the Phos-tag captures the phosphopeptide in preference to its nonphosphorylated counterpart. Binding of the phosphopeptide to the Phos-tag molecule brings the donor AMCA (λex 345 nm) close to the acceptor FAM (λem 520 nm), resulting in an efficient FRET signal. Here we introduce an application of the FRET system to the reverse reaction, phosphorylation of a FAM-labeled

 Please wait while we load your content...
Please wait while we load your content...