Towards mapping electrostatic interactions between Kdo2-lipid A and cationic antimicrobial peptides via ultraviolet photodissociation mass spectrometry†
Abstract
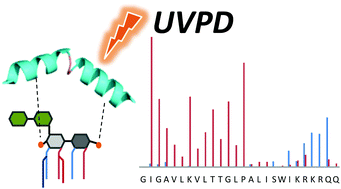
Cationic antimicrobial peptides (CAMPs) have been known to act as multi-modal weapons against Gram-negative bacteria. As a new approach to investigate the nature of the interactions between CAMPs and the surfaces of bacteria, native mass spectrometry and two MS/MS strategies (ultraviolet photodissociation (UVPD) and higher energy collisional activation (HCD)) are used to examine formation and disassembly of saccharolipid·peptide complexes. Kdo2-lipid A (KLA) is used as a model saccharolipid to evaluate complexation with a series of cationic peptides (melittin and three analogs). Collisional activation of the KLA·peptide complexes results in the disruption of electrostatic interactions, resulting in apo-sequence ions with shifts in the distribution of ions compared to the fragmentation patterns of the apo-peptides. UVPD of the KLA·peptide complexes results in both apo- and holo-sequence ions of the peptides, the latter in which the KLA remains bound to the truncated peptide fragment despite cleavage of a covalent bond of the peptide backbone. Mapping both the N- and C-terminal holo-product ions gives insight into the peptide motifs (specifically an electropositive KRKR segment and a proline residue) that are responsible for mediating the electrostatic interactions between the cationic peptides and saccharolipid.


 Please wait while we load your content...
Please wait while we load your content...