Cavity enhanced liquid-phase stopped-flow kinetics
Abstract
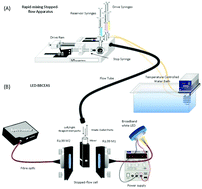
The first application of liquid-phase broadband cavity enhanced spectroscopy (BBCEAS) to the measurement of stopped-flow kinetics is reported. The stopped-flow technique is widely used for the study of the kinetics of fast liquid-phase reactions down to millisecond timescales. UV-visible absorption spectroscopy is commonly used as the detection method. Increased sensitivity can potentially allow reactions which are too fast to be measured, to be studied by slowing down the reaction rate through the use of lower concentration of reactants. A simple low cost BBCEAS experimental setup was coupled to a commercial stopped-flow instrument. Comparative standard absorption measurements were also made using a UV-visible double-beam spectrometer as the detector. Measurements were made on the reaction of potassium ferricyanide with sodium ascorbate under pseudo-first order conditions at pH 8 and pH 9.2 A cavity enhancement factor (CEF) of 78 at 434 nm was obtained whilst the minimum detectable change in the absorption coefficient αmin(t), was 1.35 × 10−5 cm−1 Hz−1/2. The kinetic data at pH 9.2 was too fast to be measured using conventional spectroscopy, whilst the BBCEAS measurements allowed 30 fold lower concentration of reactants to be used which slowed down the reaction rate enough to allow the rate constant to be determined. The BBCEAS results showed a 58 fold improvement in sensitivity over the conventional measurements and also compared favourably with the relatively few previous liquid-phase cavity enhanced kinetic studies which have been performed using significantly more complex and expensive experimental setups.


 Please wait while we load your content...
Please wait while we load your content...