Fluorescence light-up detection of cyanide in water based on cyclization reaction followed by ESIPT and AIEE†
Abstract
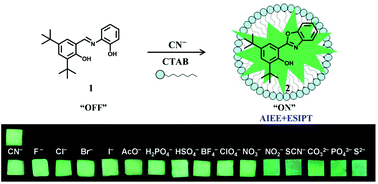
Schiff base 1 (2,4-di-tert-butyl-6-((2-hydroxyphenyl-imino)-methyl)phenol) containing two hydroxyl groups could undergo an oxidative cyclization reaction and then generate hydroxyphenylbenzoxazole (HBO) 2 when CN− was present as a catalyst. The multistep cyclization reaction was proved by spectroscopy, 1H NMR, 13C NMR and mass spectra. C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization is the predominant decay process of the excited states, so sensor 1 is weakly emissive in solution at ambient temperature. When 1 reacts with CN−, the emission is remarkably enhanced, where 1 is converted to 2. The cyclization product HBO 2 displays bright green luminescence in micellar due to the ESIPT (excited-state intramolecular proton transfer) as well as AIEE (aggregation-induced emission enhancement) effect. The detection limit is 5.92 × 10−7 M, lower than the WHO guideline of CN− in drinking water (1.9 μM). The selective and competitive experiments reveal that sensor 1 shows high sensing selectivity and sensitivity for CN− over other anions. Test papers containing absorbed 1 were prepared and applied for practical application of cyanide detection.
N isomerization is the predominant decay process of the excited states, so sensor 1 is weakly emissive in solution at ambient temperature. When 1 reacts with CN−, the emission is remarkably enhanced, where 1 is converted to 2. The cyclization product HBO 2 displays bright green luminescence in micellar due to the ESIPT (excited-state intramolecular proton transfer) as well as AIEE (aggregation-induced emission enhancement) effect. The detection limit is 5.92 × 10−7 M, lower than the WHO guideline of CN− in drinking water (1.9 μM). The selective and competitive experiments reveal that sensor 1 shows high sensing selectivity and sensitivity for CN− over other anions. Test papers containing absorbed 1 were prepared and applied for practical application of cyanide detection.


 Please wait while we load your content...
Please wait while we load your content...