Ion concentration polarization for pre-concentration of biological samples without pH change†
Abstract
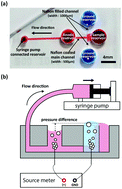
In this paper, a method was developed for pre-concentrating large-volume biological samples for subsequent analysis. We previously developed another pre-concentration device, but it unfortunately altered the pH of the sample when an electric field was applied to the sample reservoir. Changes in the pH are not suitable for subsequent antibody–antigen reactions because of the stability issues that arise based on the target molecule's isoelectric point (pI). Here, this problem was overcome using ion concentration polarization (ICP) with a cation selective membrane (Nafion). Phosphate buffered saline was used as a test solution for the sample. The sample was contained in a reservoir that was not affected by the electric field, and an ICP barrier was formed in front of the reservoir. This device could concentrate microliter-scale samples without changing the pH because the biomolecules were blocked from passing through the ICP barrier while the sample (phosphate buffered saline) was drained. A 40 μL sample was successfully pre-concentrated to 20 μL in a single channel device and 10 μL in a dual channel device, resulting in 2.1-fold and 3.3-fold increases, respectively, in influenza hemagglutinin concentrations. These changes in the concentration were confirmed by ELISA.

 Please wait while we load your content...
Please wait while we load your content...