Tandem differential mobility spectrometry with ion dissociation in air at ambient pressure and temperature
Abstract
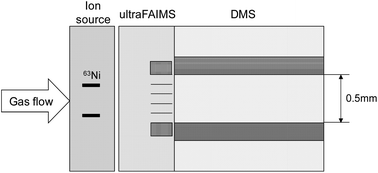
Proton-bound dimers were dissociated to protonated monomers in air at ambient pressure and temperature using electric fields of ultrahigh Field Asymmetric Ion Mobility Spectrometry (ultraFAIMS) with the onset of dissociation for ethyl acetate as 96 Td and for dimethyl methyl phosphonate as 170 Td. Ions then were measured by differential mobility spectrometry (DMS). Fragment ions were formed with propyl acetate at electric fields of 90 Td or greater. The dissociation in ultraFAIMS of ions, with compensation fields near zero, to form smaller ions with new compensation fields, provided a method to improve peak capacity in DMS without gas modifiers. These findings also lay the foundation for a triple stage DMS with a centre stage for ion dissociation or fragmentation.

 Please wait while we load your content...
Please wait while we load your content...