A depropargylation-triggered fluorescence “turn-on” probe for the detection of Pd2+ based on a bispropargylamine–rhodamine conjugate†
Abstract
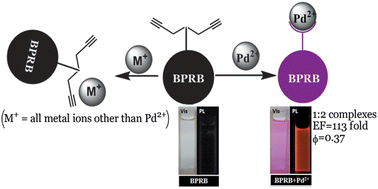
A bis-propargyl-appended rhodamine B-based receptor BPRB has been synthesised that exhibits pronounced fluorescence enhancement in the presence of Pd2+ ions. The addition of Pd2+ enhanced the fluorescence intensity of BPRB by 113-fold (Φf = 0.37) and BPRB was found to exhibit high selectivity towards Pd2+ compared to a range of other metal ions. The enhancement of fluorescence was triggered by spirolactam ring opening followed by depropargylation of BPRB in the presence of Pd2+, as evidenced by

 Please wait while we load your content...
Please wait while we load your content...