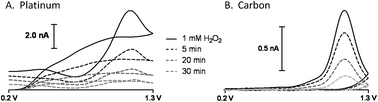
Hydrogen peroxide (H2O2) is a critically important signaling molecule. Endogenous H2O2 mediates diverse physiological processes both intra- and intercellularly; and enzymatically generated H2O2 is a widely used reporter molecule at biosensors that rely on enzymes to detect non-electroactive species. However, the development and application of electroanalytical methods for the direct detection of this molecule has been challenging because the electron transfer kinetics for the irreversible oxidation of H2O2 are slow. We comparatively characterize the electrochemical oxidation of H2O2 on bare and Nafion®-coated platinum and carbon-fiber microdisc electrodes using fast-scan cyclic voltammetry (FSCV). Using a waveform ranging from +0.2 to +1.3 V at 400 V s−1, the electrocatalytic properties of the platinum surface were not readily apparent, and the carbon-fiber microelectrode demonstrated greater sensitivity and selectivity toward H2O2. Nafion®-coating further enhanced detection on carbon electrodes. These results confirm that platinum electrodes, with or without Nafion®, will not work acceptably with this approach, and confirm the value of carbon-fiber microelectrodes relative to more traditionally used platinum electrodes in the direct detection of rapid H2O2 fluctuations using FSCV.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...