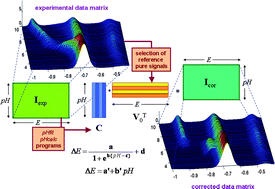
A new chemometric approach is put forward, dealing with the non-linear behaviour observed in the multivariate curve resolution (MCR) analysis of certain overlapping voltammetric signals obtained in titrations of metal complexes where pH is progressively changed. In such cases, non-reversible reduction signals move along the potential axis as a consequence of the involvement of H+-ions in the electrochemical process and cause a dramatic loss of linearity, which hinders accurate MCR analysis. The method proposed is based on the least-squares fitting of peak potential vs. pH datasets to parametric linear and sigmoid functions through the decomposition of the data matrix into both a concentration profile matrix and a unit signal matrix, in a similar way as in the alternating least-squares algorithm of MCR (ALS). Such calculations are carried out through several home-made Matlab programs which are freely available as Supplementary Material of the present work. The fitted parameters, along with the evolution of resolved concentrations and potential shifts with pH, provide valuable information on the complexation/reduction processes. The method is tested first on the relatively simple Cd(II)–NTA system and then applied to the study of the binding of Cd(II)-ions by glutathione (γ-Glu-Cys-Gly, GSH) and the phytochelatin PC2 ((γ-Glu-Cys)2-Gly).
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...