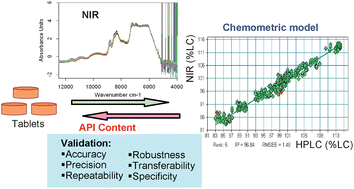
Robust NIR transmission spectroscopic methods have been developed for determination of content uniformity (CU) of pharmaceutical products with a complex tablet matrix. The tablets of interest, formulated with eight components with active drug load of approximately 30% (w/w), are non-film coated, embossed, and round with thickness values of 3.6 and 5.6 mm, for the 125 and 500 mg dosage strength, respectively. The calibration data set contained seven laboratory scale batches of tablets with concentration range of active pharmaceutical ingredients (API) varying from 85 to 115% relative to label claim (LC) as well as four full scale production batches of tablets that included the natural physical variability of tablets. The reference concentration values were established by a high performance liquid chromatographic method. Partial least-squares (PLS) regression method was used to generate the calibration models. The root mean squared error of calibration for 125 and 500 mg was 1.6 and 1.5% in LC, respectively. The calibration models were validated in terms of measurement accuracy, repeatability, precision, robustness and transferability. Robustness assessment involved challenging the model with tablets incorporating variations in hardness, excipient vendors, excipient content and excipient particle size. The methods exhibited excellent measurement accuracy based on 87 batches (ten tablets for each batch) evaluated. The transferability of the developed NIR methods was demonstrated by comparing the NIR CU results associated with the same set of tablets scanned at the development site with those scanned at the production site. The result indicates that the NIR method can be used as a suitable alternative to the HPLC method for rapid tablet CU release test in pharmaceuticals.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...