More than ring-strain: revisiting the definition of enthalpy in ring-opening polymerization†
Abstract
The thermodynamics of ring-opening polymerization (ROP) are central when predicting the chemical recyclability of aliphatic polyesters and polycarbonates. Conceptually, the enthalpy of polymerization, 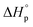 , is widely understood as a measure of ring-strain for a given monomer. However, the ring-strain is commonly larger than
, is widely understood as a measure of ring-strain for a given monomer. However, the ring-strain is commonly larger than 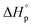 , generating the question of how the release of ring-strain energy during ring-opening transforms. In this work, we propose that
, generating the question of how the release of ring-strain energy during ring-opening transforms. In this work, we propose that 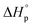 is the sum of the energy released by the ring-strain
is the sum of the energy released by the ring-strain 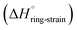 and the energy absorbed by the polymer conformations
and the energy absorbed by the polymer conformations 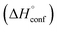 . Owing to the similar ring-strain, but vastly different
. Owing to the similar ring-strain, but vastly different 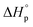 values, δ-valerolactone, δ-caprolactone, δ-octalactone, and δ-decalactone were used as model compounds to evaluate the energy cost of polymer conformational freedom. Polymer conformation, measured by 13C NMR, DSC, and molecular dynamics, results are in good agreement with the hypothesis and can explain previous literature observations i.e. positive
values, δ-valerolactone, δ-caprolactone, δ-octalactone, and δ-decalactone were used as model compounds to evaluate the energy cost of polymer conformational freedom. Polymer conformation, measured by 13C NMR, DSC, and molecular dynamics, results are in good agreement with the hypothesis and can explain previous literature observations i.e. positive 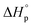 for systems with ring-strain, substituent effects, and solvent effects, that are difficult to understand when only using the analogy of ring-strain and
for systems with ring-strain, substituent effects, and solvent effects, that are difficult to understand when only using the analogy of ring-strain and 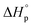 . We believe that our results provide a deeper understanding of the underlying thermodynamics and their interpretation in ROP.
. We believe that our results provide a deeper understanding of the underlying thermodynamics and their interpretation in ROP.

- This article is part of the themed collection: Polymerisation and depolymerisation chemistry: the second century


 Please wait while we load your content...
Please wait while we load your content...