Effect of salt selection and molar ratio in molten salt synthesis of single-crystalline LiNiO2†
Abstract
Single crystal (SC) layered lithium metal oxides (LiNixCoyMnzO2, referred to as NCMs or NMCs) represent a new material design with unique benefits compared to their polycrystalline counterparts. However, preparation of SC-NCMs remains challenging, with methods such as molten salt synthesis being largely dependent on recipe-by-recipe reporting and no overarching conception of how to prepare desirable materials. For a model NCM, LiNiO2 (LNO), we use experimental design methodology to identify how the factors of salt selection, salt mixtures, and molar salt ratio affect the critical features of grain size, crystal defect content (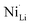 ), and particle size distribution. Herein, we identify that NaCl and KCl are salts which can be used to control particle size at relatively low defect content, while sulfate-containing salts are deleterious to quality LNO. The results of this work set out to provide a more comprehensive understanding of how others could prepare well-ordered SC-NCMs of specific particle size.
), and particle size distribution. Herein, we identify that NaCl and KCl are salts which can be used to control particle size at relatively low defect content, while sulfate-containing salts are deleterious to quality LNO. The results of this work set out to provide a more comprehensive understanding of how others could prepare well-ordered SC-NCMs of specific particle size.



 Please wait while we load your content...
Please wait while we load your content...