Carbon–metal complex as a functional material that governs the efficient conversion of Li2CO3 to LiOH·H2O†
Abstract
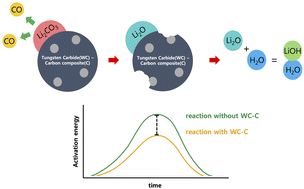
The increasing need for cathode materials comprising mainly nickel has resulted in an increased demand for lithium hydroxide (LiOH), which is a crucial precursor. LiOH can be obtained by processing minerals or converting lithium carbonate (Li2CO3). Several approaches have been proposed for implementing the latter method. Disadvantageously, most of them have low conversion efficiencies or produce residual impurities, which result in an increased manufacturing time and cost. In this study, tungsten carbide-loaded carbon black was synthesized using a simple liquid-in-plasma approach. Then, it was mixed with Li2CO3 and heat-treated, resulting in Li2CO3 being converted into lithium oxide. Finally, LiOH was dissolved in water. The conversion rate of this method approached 100%, and notably, the temperature required for conversion was lowered by about 100 °C compared to that under the method that used a general carbon source. The proposed approach is expected to provide a new environmentally- and economically-friendly route for supplying LiOH.


 Please wait while we load your content...
Please wait while we load your content...