Metal-cation-induced shifts in thiolate redox and reduced sulfur speciation†
Abstract
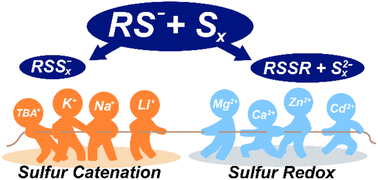
Sulfur-containing anions (e.g. thiolates, polysulfides) readily exchange in solution, making control over their solution speciation and distribution challenging. Here, we demonstrate that different redox-inactive alkali, alkaline earth, and transition metals (Li+, Na+, K+, Mg2+, Ca2+, Zn2+, and Cd2+) shift the equilibria of sulfur catenation or sulfur reduction/oxidation between thiolate, polysulfanide, and polysulfide anions in acetonitrile solution. The thermodynamic factors that govern these equilibria are examined by identification of intermediate metal thiolate and metal polysulfide species using a combination of NMR spectroscopy, electronic absorption spectroscopy, and mass spectrometry. Electrochemical measurements demonstrate that the metal cation of the electrolyte modulates both sulfur reduction and thiolate oxidation potentials. DFT calculations suggest that the changes in equilibria are driven by stronger covalent interactions between polysulfide anions and more highly charged cations.


 Please wait while we load your content...
Please wait while we load your content...