Abstract
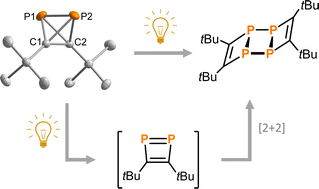
Di-tert-butyldiphosphatetrahedrane (tBuCP)2 (1) is a mixed carbon- and phosphorus-based tetrahedral molecule, isolobal to white phosphorus (P4). However, despite the fundamental significance and well-explored reactivity of the latter molecule, the precise structure of the free (tBuCP)2 molecule (1) and a detailed analysis of its electronic properties have remained elusive. Here, single-crystal X-ray structure determination of 1 at low temperature confirms the tetrahedral structure. Furthermore, quantum chemical calculations confirm that 1 is isolobal to P4 and shows a strong largely isotropic diamagnetic response in the magnetic field and thus pronounced spherical aromaticity. A spectroscopic and computational study on the photochemical reactivity reveals that diphosphatetrahedrane 1 readily dimerises to the ladderane-type phosphaalkyne tetramer (tBuCP)4 (2) under irradiation with UV light. With sufficient thermal activation energy, the dimerisation proceeds also in the dark. In both cases, an isomerisation to a 1,2-diphosphacyclobutadiene 1′ is the first step. This intermediate subsequently undergoes a [2 + 2] cycloaddition with a second 1,2-diphosphacyclobutadiene molecule to form 2. The 1,2-diphosphacyclobutadiene intermediate 1′ can be trapped chemically by N-methylmaleimide as an alternative [2 + 2] cycloaddition partner.


 Please wait while we load your content...
Please wait while we load your content...