Photochemical organocatalytic heteroarylation of anilines and secondary alicyclic amines in continuous-flow†
Abstract
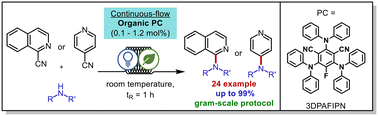
C–N bond formation plays a crucial role in various chemical synthesis processes in both the chemical industry and nature. While numerous methods have been developed for the formation of C–N bonds, only a few meet the criteria of “green chemistry”. In this study, we present a continuous-flow procedure for the decyanative N-heteroarylation of anilines and secondary alicyclic amines using a photoorganocatalytic reaction. Through the synergistic combination of photochemistry, organocatalysis, and continuous-flow technology, we were able to achieve high and close to quantitative yields, while significantly reducing the reaction time from 12 hours in batch procedures to just 1 hour in continuous-flow conditions.


 Please wait while we load your content...
Please wait while we load your content...