Novel mono substituted pyridoimidazoisoquinoliniums via a silver-catalyzed intramolecular cyclization and their applications in cellular imaging†
Abstract
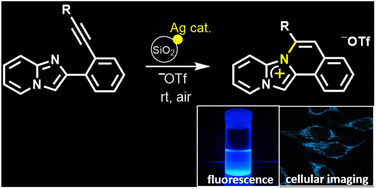
Cationic heterocycles, an important class of organic compounds soluble in polar solvents, have been gaining attention in the construction of fluorescent probes. This paper reports the quick synthesis of novel pyrido[1′,2′;2,3]imidazo[5,1-a]isoquinoliniums starting from 2-(2-ethynylphenyl)imidazo[1,2-a]pyridines at room temperature via intramolecular cyclization by employing a catalytic amount of silver trifluoromethanesulfonate in addition to lithium trifluoromethanesulfonate and silica gel as the counter anion source and additive, respectively. The designed pyridoimidazoisoquinoliniums consisted of an imidazo[1,2-a]pyridine fused isoquinolinium. The X-ray diffraction results revealed that pyrido[1′,2′;2,3]imidazo[5,1-a]isoquinolinium trifluoromethanesulfonate contained considerable planar parent skeletons and interacted by π–π stacking with neighbouring molecules. Furthermore, in a methanol solution the designed 6-phenyl derivative exhibited strong fluorescence in the 420–450 nm region in addition to strong mitochondrial specificity in a cell staining assay.


 Please wait while we load your content...
Please wait while we load your content...