Synthesis of multisubstituted carbazol-4-amines from tetrahydrocarbazol-4-one oximes†
Abstract
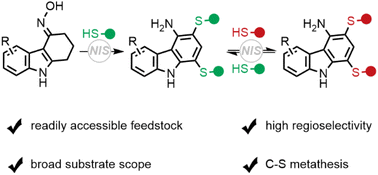
Described herein is a sequential thiolation reaction between tetrahydrocarbazol-4-one oximes and aryl thiols, affording a wide range of 1,3-dithiolated carbazol-4-amines, which are hardly accessible by other traditional methods. This reaction involving an iminyl radical intermediate features good chemoselectivity and regioselectivity, a high yield, a broad substrate scope, and easy scale-up. Moreover, under our reaction conditions, these 1,3-dithiolated carbazol-4-amines can undergo an interesting carbon–sulfur bond metathesis with other thiols.


 Please wait while we load your content...
Please wait while we load your content...