Platform for 3-fluoro-3-hydroxyoxindoles: photocatalytic C–N cross-coupling and deaminative oxidation–fluorohydroxylation†
Abstract
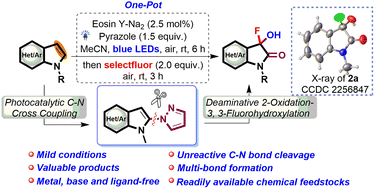
Herein, a novel and efficient protocol is developed to synthesize 3-fluoro-3-hydroxyoxindoles through a one-pot stepwise photocatalytic C–N cross-coupling and deaminative 2-oxidation–3,3-fluorohydroxylation process. This approach permits a broad range of deaminative fluorohydroxylation reactions to furnish pharmaceutically valuable fluorinated oxindoles from readily available chemical feedstocks under mild conditions. The control experiments disclosed that the removal of pyrazole-analogues of intermediate 2-pyrazolyl indoles was a key step in this transformation. In light of the pharmaceutical value of fluorinated oxindoles and the versatility of the deaminative transformation, we anticipate that this methodology will be an effective complement in the synthesis of 3-fluorooxindoles.
- This article is part of the themed collection: 2024 Organic Chemistry Frontiers HOT articles


 Please wait while we load your content...
Please wait while we load your content...