Oxidative cyclization and enzyme-free deiodination of thyroid hormones†
Abstract
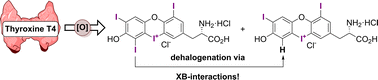
We introduce the first non-enzymatic deiodination of thyroid hormones from a so far unknown hypervalent iodaoxinium state. After developing oxidative processes for thyroxine (T4)-derived model cyclic diaryliodonium salts, we successfully produced an iodaoxinium salt through the direct oxidation of O- and N-protected T4. DFT calculations revealed a novel halogen bonding-based deiodination mechanism, circumventing the traditional selenium-dependent pathways. Our findings open new avenues in thyroid hormone chemistry, suggesting alternative mechanisms for their involvement in metabolic processes, regulation of oxidative stress, and gene expression.


 Please wait while we load your content...
Please wait while we load your content...