Modular synthesis of C2-symmetric nitrogen-containing polyaromatics via visible light-enabled reductive aza-6π electrocyclization†
Abstract
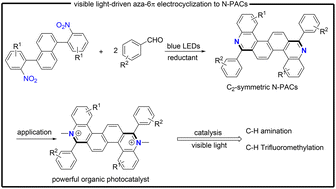
Nitrogen-containing polyaromatic compounds (N-PACs) show wide applications in synthetic chemistry and materials science, but their highly efficient preparation with structurally precise control is a big challenge. Herein, we report an unprecedented one-step modular construction of C2-symmetric N-PACs through visible-light-enabled tandem two-fold direct reduction/aza-6π electrocyclization of nitroarenes with benzaldehydes. This protocol provides a facile and versatile strategy for straightforward access to diverse N-PACs with good yields and excellent functional group compatibility under mild metal-free conditions. More importantly, the resultant C2-symmetric N-PAC 3ad could be readily converted into dicationic ammonium salt Me-3ad, which proved to be a powerful organic photocatalyst for C–H amination and trifluorometlylation.


 Please wait while we load your content...
Please wait while we load your content...