Photoproperties of favipiravir and its 6-substituted analogues: fluorescence controlled through halogen substitution and tautomerism†
Abstract
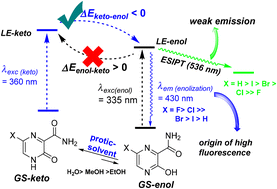
Herein, we have showed the photophysical properties of favipiravir and its 6-substituted analogues. Also, we interpreted the origin of fluorescence of favipiravir and its 6-substituted analogues as a function of tautomerism modulation in ground and excited states. Favipiravir, the 6-fluorine derivative, showed the best photophysical profile, exhibiting a dominant emission wavelength of 430 nm, a high quantum yield (Q.Y.) of 1.0 and a long-lived state (10 ns). Its analogues also showed a maximum emission at 430 nm, but their Q.Y. values were 5-fold lower than that found for favipiravir, decreasing as a function of 6-substitution as follows: F > Cl > Br > I > H. Pyrazines bearing the least electronegative 6-substituent (X = Br, I, H) showed an extra lifetime, which was shorter (0.2–0.3 ns) and less abundant (>15%) than the main lifetime (10 ns, 85%). Further 2D excitation–emission matrix and solvent studies supported that these 3-hydroxy-2-pyrazinecarboxamides present two emissive states. The first of them (λem = 430 nm), which was the most abundant, most fluorescent and long-lived state, was characterized as “locally excited” (LE). Its fluorescence was favored with an increase of the hydrogen-donor nature of the solvent and for pyrazines having a high enolic characteristic. Thus, the high LE-fluorescence of these types of pyrazines depends on the keto-tautomerization of the ground state using a protic solvent and its feasible enol-tautomerization upon excitation. Finally, the second excited state (λem = 536 nm) was suggested as an excited-state intramolecular proton-transfer (ESIPT), and it was observed only, although discretely, for pyrazines bearing the least electronegative 6-substituent.


 Please wait while we load your content...
Please wait while we load your content...