Stereoselective synthesis and antiproliferative activity of allo-gibberic acid-based 1,3-aminoalcohol regioisomers†
Abstract
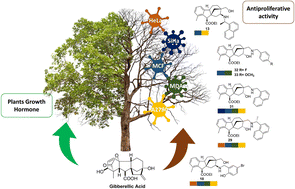
A new library of allo-gibberic acid-based aminoalcohol regioisomers was synthesised stereoselectively starting from commercially available gibberellic acid, which yields allo-gibberic acid under mild acidic conditions. The successful formation of hydroxymethyl ketone derivative 5, by acid-mediated rearrangement of previously prepared epoxide, paved the way to obtain the desired 1,3-aminoalcohols through Schiff base formation. To obtain the desired regioisomers, the primary alcohol functionality of 5 was subjected to mesylation, then replaced with either primary amine or sodium azide. The formed azide derivative was subjected to either CuAAC reaction to obtain 1,2,3-triazoles or underwent Pd-catalysed hydrogenolysis to obtain primary aminoalcohol, which was further transformed into 1,3-aminoalcohols by reductive alkylation. All prepared aminoalcohols were identified in a satisfactory manner using modern spectroscopic techniques and assessed for their antiproliferative activity against a panel of human cancer cell lines. The antiproliferative effects of the prepared compounds were assayed by in vitro MTT method against a panel of human cancer cell lines (HeLa, SiHa, A2780, MCF-7 and MDA-MB-231). A significant difference was observed in the antiproliferative activity between the regioisomers. Some compounds exerted outstanding activities against the malignant cells with limited action on fibroblasts, indicating considerable cancer selectivity.

 Please wait while we load your content...
Please wait while we load your content...