A safer, greener and faster synthesis process of sodium azide by simply altering the alcohol reactant†
Abstract
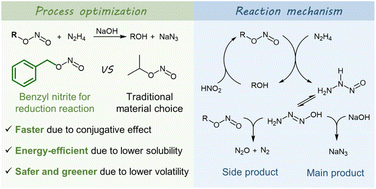
Sodium azide production suffers from safety hazards and environmental pollution rooted in the reduction reaction between alkyl nitrites and hydrazine hydrate. To enhance this heterogenous reaction, nitrites synthesized from small molecule fatty alcohols are widely used to increase its solubility in the aqueous phase. Despite these efforts, the reaction time of several hours remains necessary, and the high volatility of alkyl nitrites results in VOC emissions and safety concerns. Here, a nitrite synthesized from benzyl alcohol was used as an alternative. The aryl group provides a conjugated effect that stabilizes the transition state and reduces activation energy, thus reducing the reaction time from several hours to 20 minutes. Moreover, the higher boiling point of benzyl nitrite eliminates VOC emissions and safety problems, and the lower solubility of benzyl alcohol ensures that it can be recycled without distillation. A complete reaction mechanism was proposed based on the characterization of gaseous side products and active azo intermediates. The side reaction can be inhibited by increasing the relative concentration of hydrazine to nitrite in the organic phase. This work provides a straightforward and practical method to address potential challenges in azide production and contributes to the understanding of the aminolysis mechanism of nitrite esters.


 Please wait while we load your content...
Please wait while we load your content...