Chromium iodate: the structure and origin of optical second harmonic generation†
Abstract
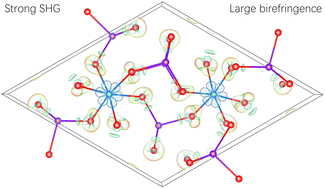
Iodate crystal materials are some of the important candidates for mid-infrared nonlinear optical (NLO) crystals. Almost all the structures and NLO properties of monometallic iodates have been studied, except for a few difficult to synthesize crystalline phases. In this work, crystalline Cr(IO3)3 was synthesized and its optical properties were studied. Cr(IO3)3 has a strong powder second-harmonic generation (SHG) effect (1.3 × AgGaS2) and a large birefringence (0.24) at 2100 nm. Theoretical calculations indicate that the 3d orbital of Cr3+ is strongly involved in the SHG process, and the contribution of the metal cation centered unit CrO6 to the SHG response is much greater than that of the non-metal cation centered unit IO3. This study provides a new case among the few NLO materials in which the SHG response is dominated by metal cation centered groups.


 Please wait while we load your content...
Please wait while we load your content...