Low-temperature synthesis of porous high-entropy (CoCrFeMnNi)3O4 spheres and their application to the reverse water–gas shift reaction as catalysts†
Abstract
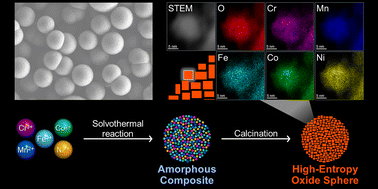
A high-entropy porous spinel oxide [(Co0.2Cr0.2Fe0.2Mn0.2Ni0.2)3O4] was synthesized via a solvothermal method and calcination. Solvothermal conditions yielding homogeneous precursor composites with five metals were optimized. Low-temperature calcination of the amorphous composites at 500 °C for 60 min yielded porous spheres formed by small primary particles, with crystal structures attributed to single-phase spinels. The homogeneity of the five elements in the spheres was verified via scanning transmission electron microscopy and energy-dispersive X-ray spectroscopy analysis. The high-entropy (Co0.2Cr0.2Fe0.2Mn0.2Ni0.2)3O4 spheres exhibited superior catalytic activity and long-term stability for the reverse water–gas shift reaction at 700 °C for at least 15 h. The importance of the Cr component in stabilizing the spinel structure was demonstrated. Mn, Fe, Co, and Ni served as active sites in the reaction. The advantage of solvothermal synthesis for porous high-entropy materials was discussed.


 Please wait while we load your content...
Please wait while we load your content...