Blocking the bimolecular pathway of water oxidation electrocatalyzed by copper porphyrin with a surfactant†
Abstract
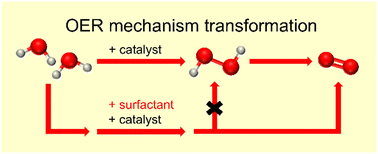
The oxygen evolution reaction (OER) is a crucial step in electrocatalytic water splitting. The formation of an O–O bond is a significant step in the OER. Herein, we report the blocking of a bimolecular pathway by adding a surfactant to copper porphyrin CuTMPyP(OTf)4 to prevent the formation of hydrogen peroxide (H2O2) in water oxidation. The addition of the negatively charged surfactant sodium dodecyl sulfonate (SDS′) to positively charged water-soluble copper porphyrin in phosphate buffer solution can efficiently change the water oxidation pathway. This work demonstrates the rare bimolecular O–O bond formation catalyzed by copper porphyrin and provides a valuable strategy for understanding and regulating the mechanism of O–O bond formation in water oxidation.


 Please wait while we load your content...
Please wait while we load your content...