Synthesis of a sacubitril precursor via the construction of one-pot chemoenzymatic cascades†
Abstract
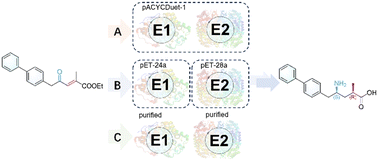
Enzymatic cascade synthesis of valuable chiral pharmaceutical intermediates has attracted attention owing to efficient and environmentally friendly routes. Currently, the synthesis of a key precursor of sacubitril valsartan sodium hydrate LCZ696 proceeds via separate steps of metal-catalyzed asymmetric hydrogenation and enzymatic transamination. Herein, we present a one-pot enzymatic cascade strategy for the construction of two chiral centers without the separation of intermediates. Upon enzyme discovery and identification, an ene-reductase GoER from Gluconobacter oxydans was incorporated in one-pot cascades with enzymatic transamination. Three different biocatalytic cascades were developed, utilizing whole cells co-expressing two enzymes, a mixture of whole cells expressing a single enzyme, and purified enzymes, affording a key intermediate in up to 87% yield and 99% de. More importantly, the scale-up preparation afforded 68.5% isolated yield with excellent diastereoselectivity. This study not only offers a sustainable alternative but also paves the way for the scalable production of a key sacubitril precursor.


 Please wait while we load your content...
Please wait while we load your content...