Supported Pd-catalyzed ring opening and chemoselective aminocarbonylative coupling of benzoxazoles with aryl iodides†
Abstract
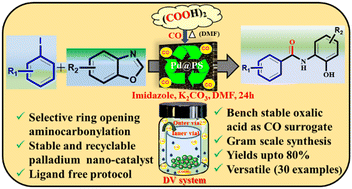
A tandem approach using polystyrene-supported palladium (Pd@PS) nanoparticles (NPs) catalyzed aminocarbonylative coupling of benzoxazole with aryl iodides to synthesize N-(2-hydroxyphenyl)benzamides has been devised. This protocol involves the ring opening of benzoxazole to 2-aminophenol followed by aminocarbonylative coupling with aryl iodides. Furthermore, the use of oxalic acid as a sustainable and inexpensive CO source, phosphine ligands-free, diverse substrate scope with appreciable yields, catalyst reusability, and gram scale applicability are important practical features.


 Please wait while we load your content...
Please wait while we load your content...