Investigation of electrocatalytic oxygen evolution reaction (OER) selectivity against methanol oxidation on stainless steel†
Abstract
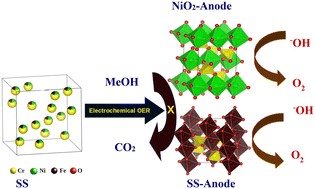
Stainless steel (SS) is a cost-effective, stable, and sustainable electrocatalyst for water oxidation. To optimize electrochemical processes, like the cathodic process of carbon dioxide reduction for methanol production, there's a critical requirement to enhance the selectivity of the oxygen evolution reaction (OER) at the anode. This improvement is necessary to effectively control the CO2 reduction product, particularly during methanol oxidation. Our recent observations showed that SS demonstrated better selectivity than the electrochemically deposited nickel oxide (EdNO) catalyst against methanol oxidation at a benchmarking current density of 10 mA cm−2 during the OER. In contrast, EdNO exhibited significant electrochemical interaction with methanol, as evidenced by the observed changes (ca. 60 mV) in open-circuit potential (OCP) with respect to methanol addition. SS did not show any electrochemical interaction with methanol under equilibrium conditions, hence there is no appreciable change in the OCP. We hypothesized that SS's high selectivity towards the OER is due to the formation of hexavalent chromium ions at the electrochemical interface, even under OCP conditions. Furthermore, unlike EdNO, the cyclic voltammogram profile of the OER at 10 mA cm−2 did not change with varying methanol concentrations when SS was used as the electrocatalyst. These findings suggest that SS could be an efficient and selective anode for carbon dioxide electrolysis to produce methanol.


 Please wait while we load your content...
Please wait while we load your content...