In situ use of photocatalytically produced H2O2 for Fenton degradation of organic pollutants
Abstract
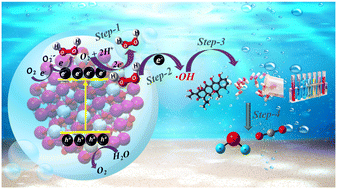
Recent years have seen increased research on green H2O2 production by photocatalysis. Though the H2O2 formation by photocatalysis is still at a dilute level, it is sufficient to degrade a given organic pollutant by a photo-Fenton process. The in situ Fenton process avoids the costs of H2O2 storage and transportation and uses it more efficiently. The present review is on the in situ Fenton use of the H2O2 formed by photocatalysis. Though this technique is called the in situ Fenton process, converting H2O2 to hydroxyl radicals (·OH) has, to date, always been achieved under light irradiation or photo-Fenton conditions. We do a thorough literature survey of the present status of such in situ Fenton reactions and classify them into three types based on the photocatalyst and the reaction system composition. The H2O2-producing photocatalyst is also the main player in the in situ Fenton generation of ·OH. Mechanism inputs are drawn from the latest photo-Fenton research to understand the in situ Fenton process better. The review ends by listing the future challenges and perspectives in this nascent area of research.
- This article is part of the themed collection: Catalysis Science & Technology Recent Review Articles, 2024


 Please wait while we load your content...
Please wait while we load your content...