Mechanistic insights into diversified photoluminescence behaviours of BF2 complexes of N-benzoyl 2-aminobenzothiazoles†
Abstract
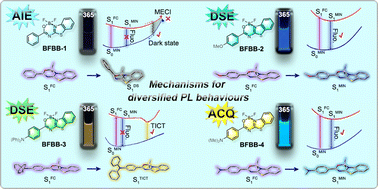
Many BF2 complexes of heteroaromatics are well known for their dual-state emission (DSE) properties. However, AIE and ACQ effects have also been observed in certain cases. To date, no rational explanations have been proposed for these uncommon photoluminescence (PL) behaviours. The current research prepared four BF2 complexes of N-benzoyl 2-aminobenzothiazoles with diversified photoluminescence (PL) properties as model compounds and utilized quantum chemical calculation tools to address this issue. Theoretical calculations revealed that the electron-donating groups (EDGs) at the para-position of the exocyclic phenyl ring exert significant influence on their ground-state electronic structures and vertical excitation features. Potential energy curve (PEC) analysis showed that the exocyclic phenyl ring and NMe2 could not function as effective rotors due to elevated energy barriers. Only the NPh2 of BFBB-3 could spontaneously rotate ∼60° to induce the formation of an emissive twisted intramolecular charge transfer (TICT) state. The two-channel model involving both vibronic relaxation and S0/S1 surface crossing revealed that the drastic narrowing of the S1/S0 energy gap in the region approaching minimun energy conical intersection (MECI) led to the generation of a dark state in BFBB-1. The small energy barrier to access the dark-state region makes the resulting fast internal conversion a competitive channel for excited-state deactivation. In contrast, the presence of EDGs in BFBB-2 and 4 inhibits this pathway, thereby resulting in intense fluorescence emissions in solution. In addition, crystallographic analysis illustrated that the F atoms perpendicular to the polyheterocycle promoted a slipped face-to-face packing mode and enhanced intermolecular interactions. The efficiencies of their solid-state emissions are mainly affected by the degree of π–π overlaps.


 Please wait while we load your content...
Please wait while we load your content...