Chemical compression and transport of hydrogen using sodium borohydride
Abstract
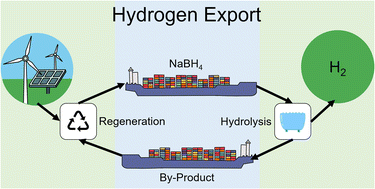
As the need for renewable energy is heightened, energy storage and distribution solutions must be developed. Hydrogen is an abundant energy source with the highest gravimetric energy density of all materials. It can be utilised in fuel cells to generate electricity, with only a water vapour by-product. For hydrogen storage and re-fuelling stations for vehicles, hydrogen compression is required to improve the volumetric energy density in storage tanks. It is proposed that sodium borohydride (NaBH4), a hydrogen carrier, could be utilised to transport and chemically compress hydrogen for refuelling stations. Chemical compression of hydrogen to over 1000 bar has been demonstrated in this study using either hydrolysis or methanolysis of NaBH4. Interest has been growing to improve the cost of closed-cycle regeneration of this borohydride energy carrier. A cost and efficiency analysis of the NaBH4 regeneration cycle using green energy demonstrates that it may be cost competitive with alternative methods of hydrogen transport, including using liquid hydrogen, ammonia, or liquid organic hydrogen carriers.
- This article is part of the themed collection: Sustainable Energy & Fuels Recent HOT Articles


 Please wait while we load your content...
Please wait while we load your content...