Controlled reductive C–C coupling of isocyanides promoted by an aluminyl anion†
Abstract
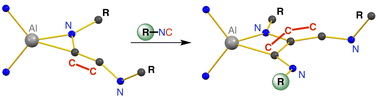
We report the reaction of the potassium aluminyl, K[Al(NON)] ([NON]2− = [O(SiMe2NDipp)2]2−, Dipp = 2,6-iPr2C6H3) with a series of isocyanide substrates (R-NC). In the case of tBu-NC, degradation of the isocyanide was observed generating an isomeric mixture of the corresponding aluminium cyanido-κC and -κN compounds, K[Al(NON)(H)(CN)]/K[Al(NON)(H)(NC)]. The reaction with 2,6-dimethylphenyl isocyanide (Dmp-NC), gave a C3-homologation product, which in addition to C–C bond formation showed dearomatisation of one of the aromatic substituents. In contrast, using adamantyl isocyanide Ad-NC allowed both the C2- and C3-homologation products to be isolated, allowing a degree of control to be exercised over the chain growth process. These data also show that the reaction proceeds through a stepwise addition, supported in this study by the synthesis of the mixed [(Ad-NC)2(Dmp-NC)]2− product. Computational analysis of the bonding within the homologised products confirm a high degree of multiple bond character in the exocyclic ketenimine units of the C2- and C3-products. In addition, the mechanism of chain growth was investigated, identifying different possible pathways leading to the observed products, and highlighting the importance of the potassium cation in formation of the initial C2-chain.
- This article is part of the themed collection: Most popular 2023 inorganic chemistry articles


 Please wait while we load your content...
Please wait while we load your content...