A donor-supported silavinylidene and silylium ylides: boroles as a flexible platform for versatile Si(ii) chemistry†
Abstract
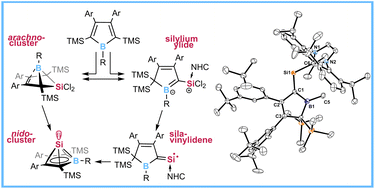
Electron-deficient, anti-aromatic 2,5-disilyl boroles are shown to be a flexibly adaptive molecular platform with regards to SiMe3 mobility in their reaction with the nucleophilic donor-stabilised precursor dichloro silylene SiCl2(IDipp). Depending on the substitution pattern, selective formation of two fundamentally different products of rivalling formation pathways is achieved. Formal addition of the dichlorosilylene gives the 5,5-dichloro-5-sila-6-borabicyclo[2.1.1]hex-2-ene derivatives. Under kinetically controlled conditions, SiCl2(IDipp) induces 1,3-trimethylsilyl migration and adds exocyclically to the generated carbene fragment giving an NHC-supported silylium ylide. In some cases interconversion between these compound classes was triggered by temperature or NHC-addition. Reduction of silaborabicyclo[2.1.1]hex-2-ene derivatives under forcing conditions gave clean access to recently described nido-type cluster Si(II) half-sandwich complexes of boroles. Reduction of a NHC-supported silylium ylide gave an unprecedented NHC-supported silavinylidene which rearranges to the nido-type cluster at elevated temperatures.


 Please wait while we load your content...
Please wait while we load your content...