Electrostatically tuned phenols: a scalable organocatalyst for transfer hydrogenation and tandem reductive alkylation of N-heteroarenes†
Abstract
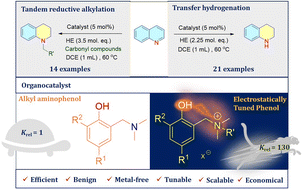
One of the fundamental aims in catalysis research is to understand what makes a certain scaffold perform better as a catalyst than another. For instance, in nature enzymes act as versatile catalysts, providing a starting point for researchers to understand how to achieve superior performance by positioning the substrate close to the catalyst using non-covalent interactions. However, translating this information to a non-biological catalyst is a challenging task. Here, we report a simple and scalable electrostatically tuned phenol (ETP) as an organocatalyst for transfer hydrogenation of N-arenes using the Hantzsch ester as a hydride source. The biomimetic catalyst (1–5 mol%) displays potential catalytic activity to prepare diverse tetrahydroquinoline derivatives with good to excellent conversion under ambient reaction conditions. Kinetic studies reveal that the ETP is 130-fold faster than the uncharged counterpart, towards completion of the reaction. Control experiments and NMR spectroscopic investigations elucidate the role of the charged environment in the catalytic transformation.
- This article is part of the themed collection: 2023 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...