Synthesis of symmetric bis-α-ketoamides from renewable starting materials and comparative study of their nucleating efficiency in PLLA†
Abstract
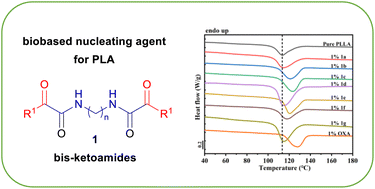
An efficient and smart synthesis of bis-α-ketoamides has been disclosed. The desired products have been obtained through a Passerini multicomponent reaction using biobased aldehydes, acetic acid and bis-isocyanides (prepared from the corresponding biobased diamides), followed by a deprotection/oxidation step. The effect of the synthesized compounds on the crystallization behavior of poly(L-lactide) (PLLA) has been investigated by differential scanning calorimetry (DSC) in non-isothermal conditions. Among all the synthesized compounds, only a few are able to meaningfully enhance the nucleation of PLLA, as confirmed by a shift of the polymer crystallization peak temperature towards higher values. With the research of active polymer nucleating agents being mostly empirical, the combinatorial synthetic approach proposed herein, coupled with the possibility of a small scale mixing procedure, can potentially represent a useful strategy for the discovery of new efficient biobased polymer additives.


 Please wait while we load your content...
Please wait while we load your content...