Total synthesis of (−)-domoic acid, a potent ionotropic glutamate receptor agonist and the key compound in oceanic harmful algal blooms†
Abstract
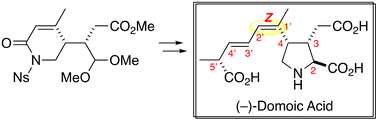
The stereo-controlled total synthesis of (−)-domoic acid is described. The critical construction of the C1′–C2′ Z-configuration was accomplished by taking advantage of an unsaturated lactam structure. The side chain fragment was introduced in the final stages of synthesis through a modified Julia–Kocieński reaction, aiming for its efficient derivatization.


 Please wait while we load your content...
Please wait while we load your content...