Synthesis, kinetics and cellular studies of new phenothiazine analogs as potent human-TLK inhibitors†‡
Abstract
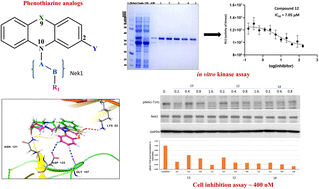
The alterations in the expression patterns of protein kinases often implicate human cancer initiation and progression. Human tousled-like kinases (TLKs), both TLK1/1B and TLK2, are evolutionary kinases found in cell signaling pathways and are involved in DNA repair, replication, and chromosomal integrity. Several reports have demonstrated the numerous roles of TLK1B in the development and progression of cancer via its interactions with different partners, and this direct association has made them viable molecular targets for cancer therapy. Previous studies have shown phenothiazines to be potent TLK1B inhibitors. Herein, we report the design and synthesis of a class of phenothiazine molecules and their biological inhibitory effect on hTLK1B/KD through in vitro kinase assays, cellular assays, and in silico studies. We identified a few inhibitors with better inhibition and physio-chemical properties than the reported TLK1B inhibitors using a recombinant human tousled-like kinase 1B-kinase domain (hTLK1B-KD). Very interestingly, inhibitory activity with LNCap cells was found to be on the sub-nanomolar level. Our attempts to study the newly designed phenothiazine analogs, as well as generate a stable catalytically active hTLK1B-KD in high yield, represent a fundamental step towards the structure-based design of future TLK-specific inhibitors.


 Please wait while we load your content...
Please wait while we load your content...