Synthesis of the ABC ring system of kadlongilactones†
Abstract
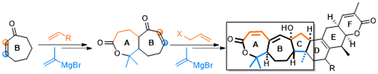
A racemic approach towards the synthesis of the ABC (7/7/5) ring system of Schisandra triterpenoid kadlongilactones is described. The synthesis features two key transformations: (1) conjugate addition followed by iodolactonization to build the cis-fused 7/7 ring; and (2) conjugate addition–Rubottom oxidation cascade followed by ring-closing metathesis to construct the 7/7/5 tricyclic ring.


 Please wait while we load your content...
Please wait while we load your content...