Experimental verification of SO2 and S desorption contributing to defect formation in MoS2 by thermal desorption spectroscopy†
Abstract
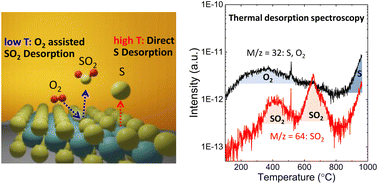
The defect-free surface of MoS2 is of high importance for applications in electronic devices. Theoretical calculations have predicted that oxidative etching could be responsible for sulfur vacancy formation. No direct experimental evidence, however, points out the role of adsorbed oxygen on sulfur vacancy formation for MoS2, especially on an insulating SiO2/Si substrate. Herein, by applying thermal desorption spectroscopy, we found that sulfur loss can be tightly coupled to adsorbed oxygen, as confirmed by observation of SO2 desorption. With annealing MoS2, even under ultrahigh vacuum, oxygen molecules adsorbed on MoS2 assist the sulfur atom in dissociating from MoS2, and thus, defects are formed as the result of SO2 desorption from 200 °C to 600 °C. At higher temperatures (over 800 °C), on the other hand, direct sulfur desorption becomes dominant. This finding can be well explained by combining the morphology investigation enabled by atomic layer deposition at defective sites and optical transitions observed by photoluminescence measurements. Moreover, a preannealing treatment prior to exfoliation was found to be an effective method to remove the adsorbed oxygen, thus preventing defect formation.


 Please wait while we load your content...
Please wait while we load your content...