Ultrasensitive low-probe-concentration PANC-1 and MCF-7 cancer cell sensors enabled by combined 2D-material-polymer-phage frameworks†
Abstract
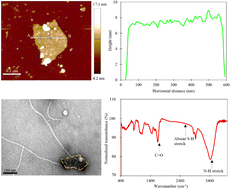
Unique biophysical properties of cancer cells can provide essential knowledge that can aid in the diagnosis of disease, clinical trial designs and drug developments. The collective response of cancer cells is of significant interest to the research community. However, sensing cells in a medium population is extremely challenging. Here, we controlled the electrical conduction of cancer cells by using combined 2D-material-polymer-phage frameworks via current flow effects. We then developed an AC-pulse sensor system based on MoS2/PEG/M13 nanoprobes for accurate detection of clinically relevant cancer cells such as PANC-1 cells and MCF-7 cells. The detection limit can reach ∼15 cells per µL, which is below an average of ∼45 cells per µL for existing sensing methods with medium cell populations. Moreover, excellent cell viability was achieved in PANC-1 cells for nanoprobe concentrations of up to 50 vol%, which surpassed those used in detection experiments. Furthermore, a nanoprobe concentration of ∼10 vol%/7.1 nM was achieved, below an average of ∼195 nM used for state-of-the-art sensing methods with medium cell populations. The combination of a previously unreported exotic sensing material and an AC-pulse strategy represents an approach for unlocking the ultrasensitive detection of cancer cells and provides a promising avenue for early cancer diagnosis, staging and monitoring.


 Please wait while we load your content...
Please wait while we load your content...