Coupled immobilized bi-enzymatic flow reactor employing cofactor regeneration of NAD+ using a thermophilic aldehyde dehydrogenase and lactate dehydrogenase†
Abstract
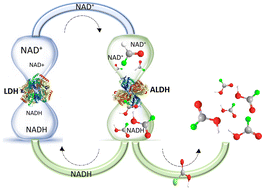
The use of enzymes in biochemical processes is of interest due to their ability to work under mild conditions while attaining high reaction rates. A limitation in the use of enzymes such as oxidoreductases on a large scale lies with their requirement for costly cofactors, e.g. NAD+, in stoichiometric quantities. Cofactor regeneration mechanisms using bienzymatic recycling systems is an attractive way to increase productivity and efficiency. The thermophilic enzyme aldehyde dehydrogenase (ALDHTt) was immobilized directly from E. coli cell lysate, containing the expressed enzyme, onto Ni2+ activated Sepharose®. The system displayed a rate of conversion of approx. 63% NAD+ with reuse achievable for up to 5 cycles and residual activity of the enzyme upon storage of 93% after 7 days. L-Lactate dehydrogenase was immobilized in a second reactor module downstream of ALDHTtvia two different methods, electrochemical entrapment in poly(3,4-ethylenedioxypyrrole) (PEDOP) and covalent attachment on glyoxyl agarose. Both reactors allowed for up to 100% conversion of NADH, however LDH@agarose proved superior in terms of reuse and storage. LDH@agarose displayed no reduction in activity after 6 cycles of use and retained 98% activity following 56 days storage. A coupled reactor containing immobilized ALDHTt–LDH was operated with the substrates hexanal, benzaldehyde, terephthalaldehyde and p-tolualdehyde. A particular advantage of the system is its ability to preferentially oxidise a single aldehyde group in substrates containing two aldehyde functional groups. The reactor demonstrated efficient cofactor regeneration under continual operation for up 24 h, with enhanced product yields.


 Please wait while we load your content...
Please wait while we load your content...