In-water oxidative Suzuki coupling of arenes and arylboronic acids using H2O2 as a terminal oxidant†
Abstract
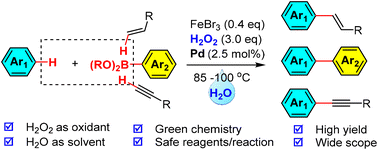
The Suzuki coupling of haloarenes with arylboronic acids is one of the most important C–C bond-forming reactions in organic chemistry. The use of arenes instead of pre-functionalized haloarenes for the (oxidative) coupling was believed to be an attractive green alternative to the classical Suzuki reaction. However, most oxidative Suzuki reactions are performed in organic solvents at high temperatures, which are operationally dangerous [three elements required for combustion: oxidant, heat, and fuel (organic solvent), are unavoidably present] and environmentally unfriendly. Herein, we report a mild oxidative Suzuki reaction in water using hydrogen peroxide as the terminal oxidant for the first time. The elimination of flammable organic solvents from the reaction prevents possible fires and explosions (starving the solvent) while the use of hydrogen peroxide as the oxidant generates no oxidant-derived waste but water as the only byproduct, both of which, represent a significant achievement from the perspective of green chemistry. By merging our Fenton-bromide system (in water) with the Suzuki reaction (in water), this new formal oxidative Suzuki coupling reaction was demonstrated with 34 examples and tolerated with many common functional groups. In addition, we showcase the extension of this strategy to two cross-dehydrogenative couplings (oxidative Heck and oxidative Sonogashira), which further expands the utility of this green strategy in organic synthesis.


 Please wait while we load your content...
Please wait while we load your content...