Anaerobic dissolved As(iii) removal from metal-polluted waters by cathode-stabilized Fe(iii)-oxyhydroxides†
Abstract
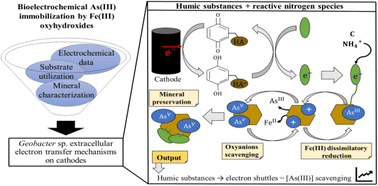
A bioelectrochemical system (BES) to efficiently induce arsenite scavenging from anoxic waters is yet to be developed. Here we examined to what extent the presence of redox reactive humic substances derivatives and reactive nitrogen species interferes with the bioelectrochemically induced immobilization of As(III) by Fe(III) oxyhydroxides. Insights from extracellular electron transfer to insoluble minerals by a strain of Geobacter sp. were acquired and integrated with data from acetate utilization. We furthered our interpretations with in situ synchrotron-based analyses of experimentally precipitated biominerals. Geobacter sp. cells interacting with cathodes used oxidized humic substance derivatives as electron shuttles which fostered the partial reduction of Fe(III), thus promoting the scavenging of As(III) oxyanions. The oxyanions became immobilized in the reactive surfaces of FeOOH within mineral aggregates, where they were readily oxidized presumably as the result of related Fenton-like reactions. An experiment lacking humic derivatives fueled the formation of bacterial–mineral networks. These networks fostered short-range electron transfer mechanisms that initially promoted biotic amorphous ferrihydrite aggregation. The early ferrihydrite aggregates exhibited a decreased As(III) scavenging capacity. In the presence of both humic derivatives and ammonium, the proposed BES was proven more effective in removing As(III) from solution and despite elevated competing phosphate levels. In the presence of reactive N species alone, stabilization of Fe(III) and microbial attachment promoted Fe(II) scavenging which outcompeted As(III) from the available ligands in the reactive mineral surfaces. Improving mineral stabilization is deemed crucial for direct As(III)-sequestration in BESs. An optimized BES for As-removal would be beneficial not only for sequestering arsenite out of solution in geogenically polluted aqueous systems, but also for addressing the recurrent eutrophication of continental water bodies linked to seasonal phosphate solubilization.


 Please wait while we load your content...
Please wait while we load your content...