Degradation of sulpiride in water by the UV/chlorine process: kinetics, reaction mechanism, and transformation pathways†
Abstract
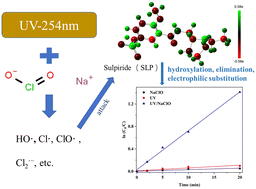
The ultraviolet/chlorine (UV/chlorine) process is a newly developed advanced oxidation process that can generate various reactive species such as hydroxyl radicals (HO˙) and reactive chlorine species (RCSs) for eliminating micropollutants in water. In this study, we investigated the kinetics and reaction mechanism of sulpiride (SLP) degradation by a UV/chlorine system, and the influencing factors, including pH, isopropyl alcohol (t-BuOH), and Br−, of SLP degradation were evaluated. SLP degradation followed the second-order kinetic model, and the rate constant of SLP degradation by the UV/chlorine process was in the range of 2.49–4.72 (mol L−1)−1 min−1. More efficient SLP degradation can be found in the UV/chlorine process than in single UV photolysis or chlorine alone. The degradation of SLP by the UV/chlorine process was more favored in acidic solution than in basic solution, which is mainly attributed to the pH-dependent dissociation between HClO and SLP. Both t-BuOH and Br− suppressed the SLP degradation by quenching radical species in the UV/chlorine process. Eight degradation intermediates were identified by ultra-performance liquid chromatography–tandem mass spectrometry (UPLC-MS/MS), and six conceivable transformation pathways were put forward. The reaction mechanism of SLP included hydroxylation, electrophilic substitution, and elimination. It showed that the combination of UV and Cl is an effective approach for SLP removal in the aquatic environment.


 Please wait while we load your content...
Please wait while we load your content...