Coupling electrocatalytic cathodic nitrate reduction with anodic formaldehyde oxidation at ultra-low potential over Cu2O†
Abstract
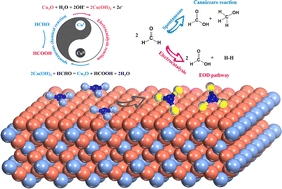
Electrocatalytic ammonia (NH3) synthesis from nitrate (NO3−) is a promising alternative to the Haber–Bosch route that requires high energy input and carbon emissions. However, sluggish anodic oxygen evolution reaction (OER) kinetics requires a large overpotential (>1.23 V vs. RHE), severely restricting the electrocatalytic cathodic NO3− reduction reaction (NO3−RR). Herein, a HCHO oxidation reaction (FOR) was developed to achieve 300 mA cm−2 at 0.81 VRHE, which was 1.56 V lower than that of the OER (Pt). A superior HCOOH production rate of 9.64 mmol cm−2 h−1 was achieved over Cu2O, approaching the highest performance reported to date. Further investigation indicated that the anodic HCHO oxidation mechanism involves electrocatalytic oxidative dehydrogenation (EOD) and tandem reaction pathways. The tandem reaction features the electrocatalytic oxidation of cubic Cu2O to orthorhombic Cu(OH)2 and spontaneous reduction of Cu(OH)2 to Cu2O by HCHO. Subsequently, the two-electrode coupling FOR and NO3−RR wherein Cu2O is utilized at both anode and cathode requires an ultra-low cell voltage of −0.19 V to achieve 10 mA cm−2 and realizes the high faradaic efficiency of 99.77% for NO3− conversion to NH3. This strategy represents a novel transformative system for simultaneously treating pollutants (HCHO and NO3−) and yielding value-added chemicals (HCOOH and NH3).


 Please wait while we load your content...
Please wait while we load your content...