Electrode/electrolyte interphases in high-temperature batteries: a review
Abstract
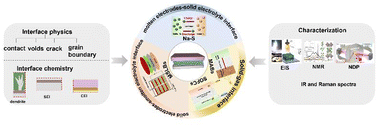
High-temperature batteries (HTBs) have attracted intensive attention due to their enhanced thermal stability and power density. To solve their main challenge of faster side reaction kinetics caused by high temperature, it is necessary to perform fundamental studies on interfacial mechanisms to further improve their performances. However, as a sealed system, it is difficult to observe the interfacial behaviors, especially in a high temperature environment. In this review, we provide full-scale characterization methods to deeply explore and understand the mechanisms of ionic transfer and interphase formation in HTBs. Then, we present a discussion about how the interphase is formed in HTBs and how the ions are transferred at three kinds of representative interfaces: liquid/solid, solid/liquid and solid gas. We also compare the corresponding interfacial processes in different electrolytes in order to figure out the influence of electrolyte components on ionic transfer mechanisms at interfaces. Based on the discussions, we can construct a narrative about the interfacial behaviors of HTBs and provide accessible methods from the interfacial perspective, which will provide many insights for effectively resolving the challenges faced by HTBs.


 Please wait while we load your content...
Please wait while we load your content...