Interfacial nanobubbles’ growth at the initial stage of electrocatalytic hydrogen evolution†
Abstract
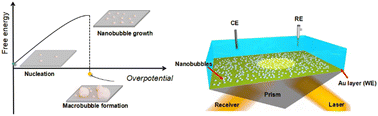
Bubble evolution in electrolysis, commonly originating from nanobubbles (NBs), usually gives rise to the extra overpotential due to a high internal pressure generated in these ultrasmall sized bubbles. The study on the growth of interfacial NBs is crucial, but nanobubble evolution during electrolysis is still vague. Herein, we employ the in situ electrochemical surface plasmon resonance imaging method, combined with atomic force microscopy measurement, to visualize the formation and growth of interfacial NBs during the initial stage of the hydrogen evolution reaction. We find that NB growth originates from pancake shaped ones, followed by increasing the coverage and roughly pinned three-phase boundaries, increasing the contact angle and height; but the coverage remains almost unchanged after reaching the equilibrium state. Further increasing the overpotential leads to the increase of the NB curvature (potential shift), as well as a higher gas outflux rate, namely, higher background current. As confirmed by molecular dynamics simulations, the “pin-rise” growth mode and the quantitative influence of NBs on the electrochemical performance have been revealed.


 Please wait while we load your content...
Please wait while we load your content...