Steric and electronic effects of arsa-Buchwald ligands on Suzuki–Miyaura coupling reaction†
Abstract
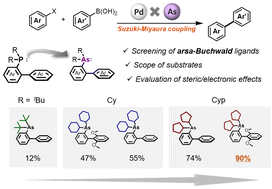
The Suzuki–Miyaura coupling (SMC) reaction is one of the most commonly used cross-coupling reactions. Bulky biaryldialkyl monophosphine ligands, i.e., Buchwald ligands, are beneficial for the SMC reaction. We recently developed a synthetic procedure for arsa-Buchwald ligands, arsenic analogs of Buchwald ligands, and found that these ligands are effective for sterically hindered substrates because of facilitating the transmetalation step owing to the longer arsenic–palladium bond. However, the relationship between the structure and steric/electronic properties of the arsa-Buchwald ligands has not yet been studied in detail. In this study, a series of arsa-Buchwald ligands with various alkyl substituents were synthesized. The cyclopentyl group afforded the highest catalytic activity for the SMC reaction, particularly with sterically hindered substrates. Furthermore, the steric/electronic properties of the arsa-Buchwald ligands were computationally analyzed.


 Please wait while we load your content...
Please wait while we load your content...